how to find the limiting reactant
Do you know !
You can easily find excess and limiting reactants
Firstly , you must know that !!
What is Limiting reactant and excess reactant?
Limiting Reactant Definition:
The reactant which is used up completely and earlier in a chemical reaction is called limiting reactant.
Limiting reactant is also known as limiting reagent .
Excess Reactant Definition:
The reagent / reactant which left behind after the completion of a chemical reaction is called excess reactant or excess reagent.

Super Simple Trick to identify Limiting & Excess Reagent:
1.Calculate the number of moles of each reactant.
2.Then calculate the total number of moles for each reactant using balanced chemical equation.
3.The reactant /reagent that gives the least amount of product is the limiting reactant & the reactant /reagent that gives the greater amount of product is the excess reactant .
Limiting reagent and Excess Reagent Example:
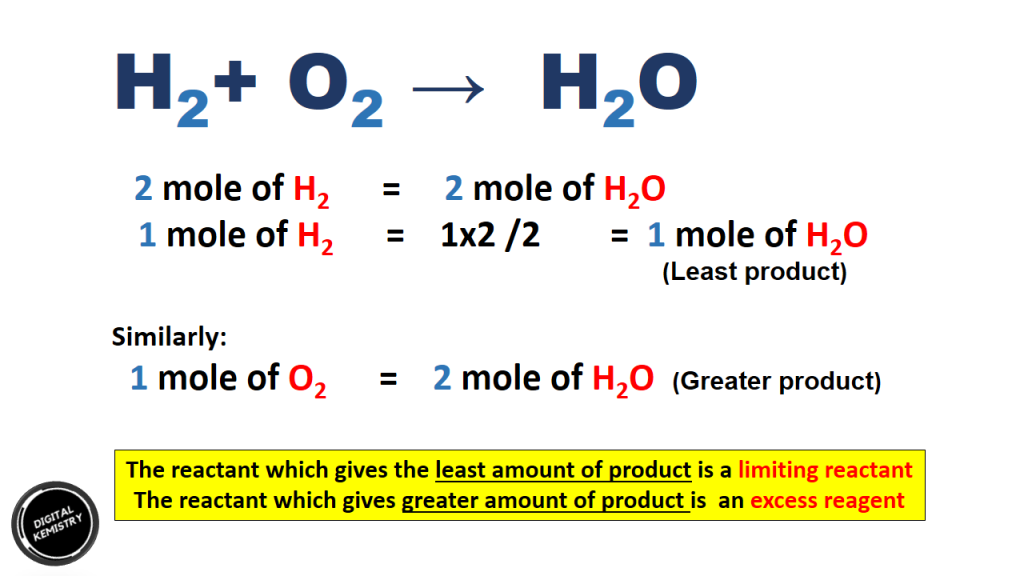
Let 1 mole of H2 & 1 mole of O2 were reacted to produce H20 . The limiting and excess reagent can be determined as:
Solution:
Balanced Chemical equation is as follow:

Given:
Unbalance Chemical Equation

i) 2 moles of H2 = 2 moles of H2O
1 moles of H2 = 1 x 2 moles of H2O = 1 moles of H2O
2
ii) 1 moles of H2 = 2 moles of H2O
Since H2 produces least amount of product therefore , it is a limiting reagent whereas O2 produces greater amount of product therefore , O2 is an excess reagent.
Determination of Excess reactant ( reagent ) consumed and un-used ( left over) in a Chemical reaction:
O 2 consumed in a chemical reaction can be easily determined by the comparison balanced and given chemical reactions.
2 mol H2 needs = 1 mol O 2 ( Balance chemical reaction)
1 mol H2 needs = 1 X 1
2
1 mol H2 needs =0.5 mol O 2
Since 0.5 moles of O2 react with 1 mole of H2 to produce H2O .
How do you find the excess reactant left over in a reaction?
Un-used ( left over) Excess reagent in a Chemical reaction:
Left over (unused) reactant = total excess reagent- amount used
Left over O2 = 1 mol – 0.5 mol
= 0.5 mol

Remember !!!

Related Important Questions:
How does a limiting reagent control the amount of product formed?
When the limiting reagent is used up completely , the reaction stops and no more product will formed.
The amount of product is always calculated on yhe basis of limiting reactant (reagent).
Limiting reactant always gives the least amount of product formation. Therefore , it controls the overall yield ( amount of product) of a chemical reaction.
Simply , the maximum amount of product formation ( yield ) depends on the amount of limiting reactant.
What is Limiting reagent?
The reactant which is earlier consumed in a reaction or The reactant which is completely consumed in a reaction to produce product when the reaction is over" is called limiting reactant.
Recommended video:
Categories: basic chemistry, chemistry, fundamentals of chemistry, fundamentals of chemistry, Stoichiometry
Tagged as: basic concepts of chemistry class 11, chemistry, class 11 chemistry excess and limiting reactants, Determining the Limiting reactant and Excess Reactant, digital kemistry, How do you find the excess reactant left over, How to find Excess Reactant and Limiting Reactant, how to find limiting reagent and excess reagent, important for neet, Limiting Reactant, limiting reactant definition, limiting reactant examples, limiting reagent numericals class 11, Limiting reagent stoichiometry, my digital kemistry, Stoichiometry, youtube
how to find the limiting reactant
Source: https://mydigitalkemistry.com/2021/07/05/how-to-find-excess-and-limiting-reactants-reagents-chemistry/
Posted by: shoafauncaughbove.blogspot.com

0 Response to "how to find the limiting reactant"
Post a Comment